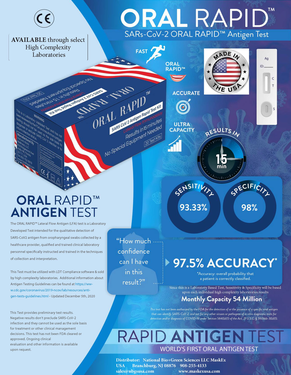
The 15 Minute SARs-CoV-2 Oral Rapid™ Antigen Test Device
|
The 15 Minute SARs-CoV-2 Oral Rapid™ Antigen Test Device is a laboratory-based Antigen Test (LDT)and is Made in the USA. It is legally approved to be sold and distributed in the USA with its sibling approved in 31 countries worldwide with a CE Mark. Only high complexity laboratories can distribute this device in the USA. All laboratories must validate our 15 Minute SARs-CoV-2 Oral Rapid ™ Antigen Test using the Laboratory Developed (LDT) pathway which is controlled by the U.S. Dept. of Health and Human Services (HHS) and permits the lawful sale of our SARs-CoV-2 Oral Rapid ™ Antigen Tests in the USA. This pathway was approved by HHS mid-August 2020 and then just on December 5th, 2020 was adopted by the CDC when the FDA formerly "authorized laboratory-based antigen tests". This guidance is very detailed and is an essential requirement to help guide employer groups, healthcare institutions and states to conduct front line testing 100% via Antigen Tests. Confirmation testing occurs approximately 6% to 15% by PCR. Our Company is independently seeking an EUA authorization via the second pathway which is controlled by the FDA. Separate validation studies are being done in order to file a 510k submission in conjunction with our EUA as soon as those independent studies are completed.
Since this is a much more advanced and lengthier validation approach, it will take some extra time at which time we will request a Full FDA approval in conjunction with an EUA authorization. We will then be the only IVD manufacturer in the world with such an approval. We have many American Companies who have purchased the tests and then compared the results to PCR to conduct their own validation studies with their employees. Some of the largest companies in the world (Publicly traded, S&P rated) have reviewed the legality of our test with their legal counsel and moved forward with their own validations which led to them purchasing our test via our partner high complexity laboratories. Currently, several national high complexity laboratories are conducting independent validations and technical refinements under our licensed brand, Oral Rapid™. In addition, two national staffing agencies have also validated the device with the employees of their corporate clients and are actively purchasing and using the test. In Nevada, a large casino has also adopted our test. |
Internationally, we as manufacturer, independent of the USA LDT, are continuing to obtain overseas approvals. The Bahamian government is actively validating the test currently and October 21st, 2020, our Oral Rapid ™ SARs-CoV-2 Antigen Rapid Test was approved to sell in 31 countries under the CE Mark for Europe.
Since this is an American laboratory validated device, as a distributor, we have taken extensive measures to ensure that our 15 Minute SARs-CoV-2 Oral Rapid ™ Antigen Test Device is fully compliant with all applicable government regulatory authorities; such as, HIPAA, CDC, HHS, and CLIA and can be legally sold in the USA. 1. We possess a software where every single test picture is uploaded, and our laboratory director (and his team) reviews every test result and formally provides a test result diagnosis. This enables each testing site to become an extension of our high complexity laboratory(s) including full LIS (Laboratory Information System) compatibility.
2. As an add-on service, we also offer a physician of record service for test service centers or employer staffing agencies who require this national service for billing purposes.
3. We have contracted with some of the largest and most reputable medical staffing agencies in the world who are well versed with our device and are available to provide licensed medical professionals for employer organizations or testing sites who require additional services at a nominal fee included in the purchase of the device.
|
Paul Singh, MHA, BA
Chairman North American Diagnostics, LLC
Chairman North American Diagnostics, LLC
If you would like to read up on additional information on the Laboratory Developed Test pathway (LDT) please refer to the following links provided by the FDA.
FDA - Laboratory Developed Tests (LDTs)
FDA - Discussion Papers on LDTs
Federal Register - Regulatory Oversight of LDTs
You can find formal acceptance by the FDA & CDC for Laboratory Developed Tests here.
CDC - Antigen Test Guidelines
FDA - Laboratory Developed Tests (LDTs)
FDA - Discussion Papers on LDTs
Federal Register - Regulatory Oversight of LDTs
You can find formal acceptance by the FDA & CDC for Laboratory Developed Tests here.
CDC - Antigen Test Guidelines
T 848-420-0755
|
[email protected]
|
Copyright © 2020